Adults with Type 1 diabetes who meet the criteria & priorities
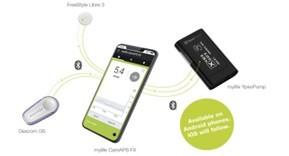
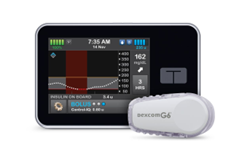

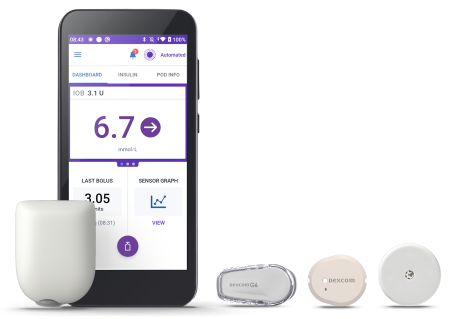
What is an insulin pump?
An insulin pump is a small electronic device which is worn on the body, it delivers fast acting insulin via a cannula under the skin. The cannula can be left in place for a few days before needing to be replaced and repositioned somewhere else on the body. An insulin pump is worn ALL the time and only taken off for bathing, swimming and contact sports.
All insulin is given through the pump. It delivers fast acting insulin in precise amounts at pre-programmed times. This is known as a basal rate and replaces the long-acting insulin. The pump user needs to be able to programme the pump and make decisions about how much insulin is required. The user needs to learn how to use the pump to deliver the required bolus (mealtime) insulin when having carbohydrates and to be able to adjust the settings in certain circumstances like physical activity, pregnancy, illness etc.
An insulin pump can be connected to certain continuous glucose monitors (CGM) to make a hybrid closed loop (HCL) system which can make some automated adjustments to insulin delivery.
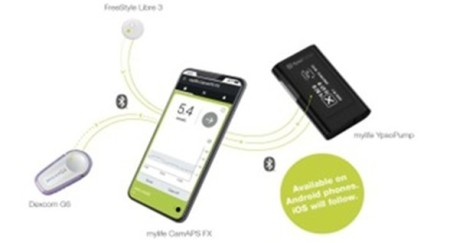
Who can have a hybrid closed loop insulin pump?
The criteria for HCL insulin pumps are set out by the National Institute for Health and Clinical Excellence (Information for the public | Hybrid closed loop systems for managing blood glucose levels in type 1 diabetes | Guidance | NICE )
There is a 5-year roll-out plan to offer a pump based on the priority list set by the Coventry & Warwickshire Integrated Care Board. Adults with type 1 diabetes can be considered if they meet the following criteria and in priority group order:
- Planning pregnancy or pregnant
- Disabling hypoglycaemia/ hypoglycaemia unawareness
- HbA1c above 58mmol/mol (starting with those with highest HbA1c deemed to consider safe to start HCL insulin pump)
In addition, the requirements prior to commencing an insulin pump are as follows:
- Attended carbohydrate counting education
- On basal bolus insulin regime
- On CGM
- Able to self-manage and adjust pump settings
- Attended retinal screening within the last 12 months: without retinopathy or stable retinopathy and currently not receiving treatment for retinopathy
If you need to attend carbohydrate counting education contact diabetes nurses on 07789946508
|
To attend retinal screening contact Arden, Herefordshire and Worcestershire Diabetic Eye Screening |
 |
| The choice of pump is based on the clinical needs. Funding for insulin pumps will be stopped if there are no clinical benefits to the individual or unable to safely manage the HCL system. This is reviewed on an ongoing basis. |
Insulin pumps: Advantages and Disadvantages
Advantages:
- Fewer injections - the needle for the cannula is only replaced a few times a week.
- More flexible insulin dose adjustment to help achieve better blood glucose control.
- May improve quality of life.
- May reduce the total dose of insulin if the diabetes control improves.
- Better glycaemic control
Disadvantages:
- Must be worn 24 hours a day, every day.
- Requires intensive education to start on a pump and constant monitoring.
- Need to check the blood glucose levels more frequently- because the insulin is short-acting and there is no long-acting insulin in the body.
- Like any device, the pump could fail, or cannulas can block which would leave the pump user without insulin and increased risk of DKA (diabetic ketoacidosis).
- Infection/allergic reaction may develop at the insertion site.
If you are interested in being referred for consideration of insulin pump therapy, please discuss with your team at your next diabetes clinic appointment.
Copyright
Image 1: Omnipod 5 with sensors. Copyrighted image used with permission. © 2025 Insulet Corporation. All rights reserved.
Ypsopump, Tandem T-Slim and Medtronic copyrighted images used with kind permission.
George Eliot Hospital is a smoke free environment. For help and advice to stop smoking you can call the national helpline on 0300 123 1044 or visit https://
Copyright
Except where otherwise noted, this item is licensed under the CC BY license (https://
If you are a rights holder and are concerned that you have found material on our patient information resources website, for which you have not given permission, or is not covered by a limitation or exception in national law, please contact us using the Feedback form providing your contact information and full details of the material.